 B-cell chronic lymphocytic leukemia (B-CLL) is the most common subtype of mature B-cell neoplasms with the overall age-standardized incidence rate equal to approx. 4 per 100 000 per year. The incidence increases rapidly with increasing age. About 70% of all CLL cases is diagnosed in the population aged 65 and over. The disorder is more common in men with a male to female ratio of approximately 1,5 : 1,1. The median survival at diagnosis varies between 1 and more than 10 years.
B-cell chronic lymphocytic leukemia (B-CLL) is the most common subtype of mature B-cell neoplasms with the overall age-standardized incidence rate equal to approx. 4 per 100 000 per year. The incidence increases rapidly with increasing age. About 70% of all CLL cases is diagnosed in the population aged 65 and over. The disorder is more common in men with a male to female ratio of approximately 1,5 : 1,1. The median survival at diagnosis varies between 1 and more than 10 years.
Many people with B-CLL do not have any symptoms when it is diagnosed. A few studies demonstrated that treatment of patients with early-stage disease does not prolong survival. Thus, the standard treatment of patients with early disease is a watch-and-wait strategy, while progressive or symptomatic disease requires therapy.
A few therapy options are currently available for symptomatic, progressive B-CLL. Usually chemotherapy or chemo / immunotherapy is applied that make use of alcylating agents (e.g. chlorambucil, bendamustine), purine nucleoside analogues (e.g. fludarabine), and / or monoclonal antibodies (e.g. alemntuzumab, rituximab) either in a monotherapy or in a combination therapy.
Efficacies of some of these treatments have been compared directly in the randomized control trials (RCTs) in terms of: the partial, complete and overall remission, the progression-free survival time (PFS) and / or overall survival (OS). However, the available data are sparse, often conflicting, and most of the therapy options have not been compared directly.
The course of B-CLL and patients response to treatment is very diverse, which make it difficult to select the most appropriate therapy option and to predict progression of the disease. While deciding about initiation of the treatment and selecting the most appropriate one from all the available schemes such parameters should be taken into consideration like: stage of the disease, the overall patient’s condition, prognostic factors, patient’s age and sex, etc. It should be also considered that in case of a relapse of the disease, which is likely in most of the patients, even though the first line treatment led to a remission, the effectiveness of the subsequent lines of treatment is often lower due to growth of the population of cells immune to the treatment.
The Nalecz Institute of Biocybernetics and Biomedical Engineering PAS in cooperation with the Clinic of Hemato-oncology and Bone Marrow Transplantation Medical University of Lublin developed and implemented a few years ago a stand-alone computer system for monitoring patients with B-CLL (BIAL). The BIAL system made it possible to collect a wide range of parameters, including those that are not routinely collected. These data were intended to be used to assess an influence of the applied treatment, including the experimental immunotherapy, on patient’s state and progression of the disease.
Based on the experience gained during an implementation of the BIAL system a need was addressed to develop a new IT system NetBIAL that should make it possible to collect, in relatively short time, a large data set originating from patients treated in many national medical centers, containing smaller group of parameters but monitored in majority of the patients with B-CLL. According to the assumptions that were made, the literature review supplemented with an analysis of the data gathered in the NetBIAL data base should enable to elaborate analytical tools for a prediction of the patient’s state and outcome of the applied treatment as well as a selection of the most appropriate therapy option.
Aim of the project covers:
- development, implementation and release of a network computer application for complex multicenter, long-term monitoring of the diagnosis, observation and treatment of patients with the B-cell chronic lymphocytic leukemia (B-CLL),
- collection of large data set concerning patients with B-CLL and containing prognostic parameters as well as parameters characterizing patient’s state, course and outcome of the applied treatment,
- an attempt to develop analytical tools to analyze the gathered data, facilitating: selection of the most effective treatment, assessment of the patient’s state and prediction of the disease progression.
During the first stage of the project in years 2010-2011, a multicenter data base of patients with B-CLL was developed together with a web-based application that enabled gathering of the data and facilitated an assessment of patient’s state and the course of the treatment, under the common name – NetBIAL system.
The NetBIAL system was implemented according to the requirements of the Inspector General for the Protection of Personal Data (GIODO) concerning the sensitive data sets (e.g. containing the data related to one’s health status). The NetBIAL system was registered in the registry of personal data sets kept by GIODO under the name “Data base of patients with chronic lymphocytic leukemia (NetBIAL)” and it was released for the use to medical partners of the project.
Currently the NetBIAL system is used for the centralized gathering of the data originating from a dozen or so Polish medical centers, in which patients with B-CLL are treated. Until Jan 1st, 2014 in the NetBIAL data base 874 patients were registered, including the data concerning 1635 started treatment lines.
The user interface of NetBIAL’s data access application was designed in such a way that reflects the procedure that the patients undergo after the diagnosis of B-CLL, i.e. determination of the patient’s state and the prognostic parameters and then usually repeating stages of: observation (monitoring), control examinations and visits, application of the selected treatment and assessment of its outcome. The system contains also a visualization module facilitating assessment of patient’s state.
An exemplary screen-shot from the NetBIAl system, showing a form concerning the applied treatment is presented below.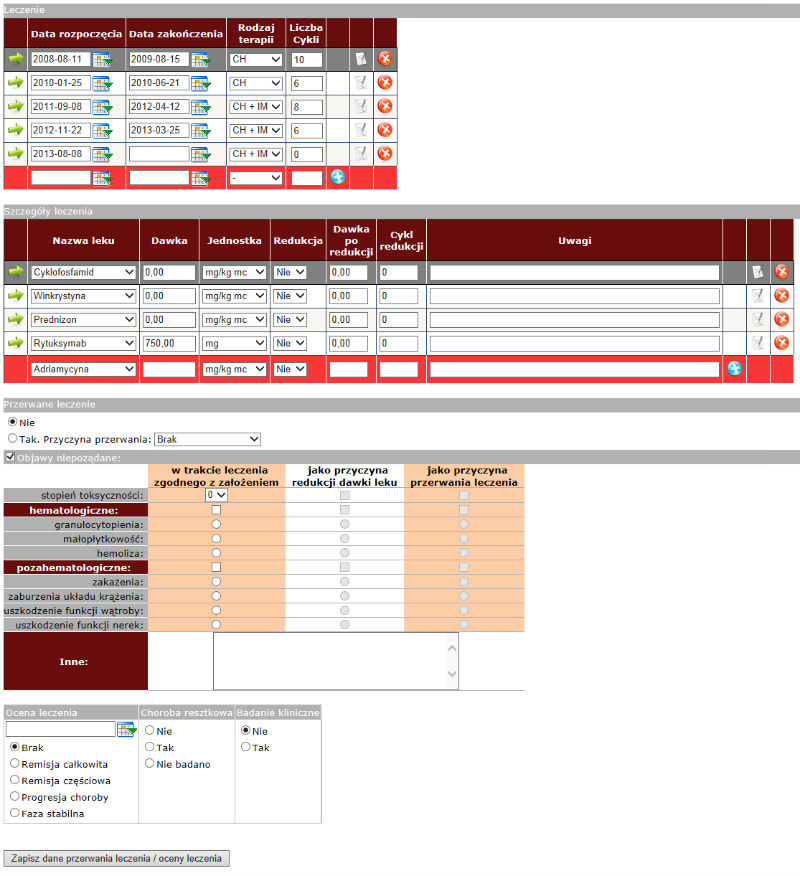
In course to develop the analytical tools enabling the prognosis of patient’s state and the outcome of the applied treatment and to facilitate the selection of the most appropriate therapy option an extensive literature review was conducted regarding the chronic lymphocytic leukemia and the multi-directional Bayesian metaanalysis was performed (using the Monte-Carlo technique) to compare different B-CLL therapy options, including:
- simple metaanalysis of the complete, partial and overall remission for two pairs of chemotherapy agents used in therapy of B-CLL,
- network metaanalysis of the complete, partial and overall remission for agents used in therapy of B-CLL with an assessment of an influence of the disease stage and patient’s age on values of these parameters,
- network metaanalysis of the progression free survival and the overall survival applying the partial polynomials to describe parameters representing the treatment effect.
This analysis was conducted using up-to-date data analysis methods, e.g. the node-splitting method to assess the consistency of the data used in the network metaanalysis or the fractional polynomials method to describe hazard function in the survival model.
The conducted analysis led to selection of models, which approximate the results of an application of the available therapy options, generalized based on the available data, in the most accurate way. The developed models / programs can be repeatedly used in future, when results of new clinical trials are published, to verify whether the most accurate models should be updated.
The last stage of the project concerns an application of the methodology related to a development of the Bayesian network to combine results of the metaanalysis, the data collected using the NetBIAL system and the medical experts’ knowledge. It should enable to utilize information concerning a particular patient (such like: risk factors, current health status or treatment history) while predicting the disease progression and while selecting the most appropriate therapy option.
Links:
- http://netbial.ibib.waw.pl (web page and service available in Polish language only)
References:
- Sant M, Allemani C, Tereanu C, De Angelis R, Capocaccia R, Visser O, Marcos-Gragera R, Maynadié M, Simonetti A, Lutz JM, Berrino F; HAEMACARE Working Group. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood. 2010;116(19):3724-34.
- National Cancer Institute, Surveillance Epidemiology and End Results. SEER Stat Fact Sheets: Chronic Lymphocytic Leukemia. http://seer.cancer.gov/statfacts/html/clyl.html , last accessed on April, 15th, 2013.
- Dighiero G, Maloum K, Desablens B, et al. Chlorambucil in indolent chronic lymphocytic leukemia. N Engl J Med. 1998;338:1506-1514.
- Shustik C, Mick R, Silver R, Sawitsky A, Rai K, Shapiro L. Treatment of early chronic lymphocytic leukemia: intermittent chlorambucil versus observation. Hematol Oncol. 1988;6:7-12.
- Montserrat E, Fontanillas M, Estape J, for the Spanish PETHEMA Group. Chronic lymphocytic leukemia treatment: an interim report of PETHEMA trials. Leuk Lymphoma. 1991;5:89-92.
- Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, Hillmen P, Keating MJ, Montserrat E, Rai KR, Kipps TJ. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008;111:5446-5456.
- Stram M, Tabarkiewicz J, Hus I, Roliński J. New approaches in treatment of B-cell chronic lymphocytic leukemia. Cancer Therapy Vol 7, 163-173, 2009.
- Eichhorst BF, Busch R, Stilgenbauer S, et al. First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood 2009;114:3382–91.
- Rai KR, Peterson BL, Appelbaum FR, et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med 2000;343:1750–7.
- Catovsky D, Richards S, Matutes E, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): a randomized controlled trial. Lancet 2007;370:230–9.
- Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomize, open-label, phase 3 trial. Lancet 2010;376:1164–74.
- Hillmen P, Skotnicki AB, Robak T, et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol 2007;25:5616–23.
- Cheng MM, Goulart B, Veenstra DL, et al. A network meta-analysis of therapies for previously untreated chronic lymphocytic leukemia. Cancer Treat Rev 2012;38:1004–11.
- Main C, Pitt M, Moxham T, Stein K. The clinical and cost-effectiveness of rituximab for the 1st line treatment of chronic lymphocytic leukaemia: an evidence review of the submission from Roche. Health Technol Assess 2010;14(Suppl. 2):27–32.
- Jansen JP. Network meta-analysis of survival data with fractional polynomials. BMC Med Res Methodol 2011;11:61.
- Julian PT Higgins, Jonathan J Deeks and Douglas G Altman (Eds.) on behalf of the Cochrane Statistical Methods Group. Special topics in statistics in Part 3 “Special topics” In Cochrane handbook for systematic reviews of interventions. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
- Lunn D, Spiegelhalter D, Thomas A, Best N. The BUGS project: Evolution, critique and future directions (with discussion). Stat Med 2009;28:3049–82.
- Spiegelhalter DJ, Best NG, Carlin BP, van der Linde A: Bayesian measures of model complexity and fit. Journal of the Royal Statistical Society, Series B 2002, 64:583-639.
- Ladyzynski P, Migdalska-Musial K, Foltynski P, Kawiak J, Hus I, Sieklucka M, Rolinski J, Dmoszynska A, Wojcicki JM: BIAL – Registration and monitoring system for patients with chronic lymphocitic leukemia. Biocybern and Biomed Eng 2005;25:57-63.
- Ładyzynski P., Migalska-Musial K, Foltynski P, Kawiak J, Hus I, Sieklucka M, Rolinski J, Dmoszynska A., Wojcicki JM: Preliminary verification of registration and monitoring system for patients with chronic lymphocytic leukemia. Proceedings of the XII Congress of Polish Society of Clinical and Experimental Immunology. Polish Journal of Environmental Studies,14 (Supl. II, part II): 629-632; 2005.
- Wojcicki JM, Ladyzynski P, Hus I, Molik M, Foltynski P, Migalska-Musia K, Kawiak J, Rolinski J, Pozarowski P, Dmoszynska A: Informatyczna platforma rejestracji i monitorowania pacjentów z przewlekłą białaczką limfocytową. I Krajowy Zjazd Polskiego Towarzystwa Cytometrii (1-st Congress of Polish Society of Cytometry), Kazimierz Dolny, 12-15 maja 2010, Abstract book, str. 15, 2010.
- Molik M, Ladyzynski P, Foltynski P, Wojcicki JM: Meta-analiza efektów pierwszej linii leczenia pacjentów z przewlekłą białaczką limfocytową B-komórkową. XVIII Krajowa Konferencja Naukowa „Biocybernetyka i Inżynieria Biomedyczna”, Gdańsk, 10-12 października, 2013, str. 1-6.
Current list of national medical units registered in NetBIAL system:
- Klinika Hematoonkologii i Transplantacji Szpiku UM w Lublinie
- Klinika Hematologii Uniwersytetu Medycznego w Łodzi
- Klinika Nowotworów Układu Chłonnego Centrum Onkologii Instytutu Marii Curie-Skłodowskiej w Warszawie
- Klinika Hematologii IHiT w Warszawie
- Klinika Hematologii, Onkologii i Chorób Wewnętrznych Warszawskiego Uniwersytetu Medycznego
- Klinika Chorób Wewnętrznych i Hematologii z Ośrodkiem Transplantacji Szpiku CSK WAM w Warszawie
- Klinika Hematologii Collegium Medicum Uniwersytetu Jagiellońskiego w Krakowie
- Klinika Hematologii i Transplantacji Szpiku Śląskiego Uniwersytetu Medycznego w Katowicach
- Klinika Chemioterapii Onkologicznej z pododdziałem leczenia chłoniaków Śląskiego Uniwersytetu Medycznego w Katowicach
- Klinika Hematologii, Nowotworów Krwi i Transplantacji Szpiku Akademii Medycznej we Wrocławiu
- Klinika Hematologii i Chorób Rozrostowych Układu Krwiotwórczego Uniwersytetu Medycznego w Poznaniu
- Klinika Hematologii i Transplantologii Gdańskiego Uniwersytetu Medycznego
- Klinika Hematologii Pomorskiego Uniwersytetu Medycznego w Szczecinie
- Oddział Wewnętrzny i Hematologii Wielospecjalistycznego Szpitala Miejskiego im. Józefa Strusia w Poznaniu
- Oddział Hematologii i Chorób Rozrostowych Układu Krwiotwórczego Szpitala Uniwersyteckiego w Bydgoszczy
- Oddział Hematologii i Chorób Wewnętrznych Wojewódzkiego Szpitala Specjalistycznego im. L. Rydygiera w Krakowie
- Oddział Hematologii Onkologicznej Szpitala Specjalistycznego Podkarpackiego Ośrodka Onkologicznego w Brzozowie
- Dział Hematologii Świętokrzyskiego Centrum Onkologii w Kielcach
- Oddział Hematologii Wojewódzkiego Szpitala Specjalistycznego im. Fryderyka Chopina w Rzeszowie
- Oddział Hematologii Specjalistycznego Szpitala Miejskiego w Toruniu
- Oddział Hematologiczny Zamojskiego Szpitala Niepublicznego w Zamościu
This list can be extended to include additional medical units, which treat patients with B-CLL and which expressed their interest to participate in the project.

 Polski
Polski English
English